Electrolyte systems play an important role in chemical engineering separations, and also in geochemical environments and for biophysics. The mean ionic activity coefficients quantify the deviation of salt chemical potential from ideal solution behavior; experimental measurements are available for many salts over broad ranges of concentration and temperature, but there have been practically no prior simulation studies of these quantities, because if sampling difficulties for explicit-solvent electrolyte solutions. We are developing new methods for determination of properties of aqueous electrolytes and using them to improve the models for water and ions, by incorporating polarizability in the intermolecular potential models. For example, we have recently used forward-flux-sampling and metadynamics methods to obtain insights on the nucleation rates and pathways for salt crystallization from supersaturated aqueous solutions and to compare different salt and water models with respect to their ability to describe experimental measurements, as shown in the figure. We are also exploring the properties of molten carbonate electrolytes, which have applications to high-temperature fuel cells that can be used to separate CO2 for carbon sequestration.
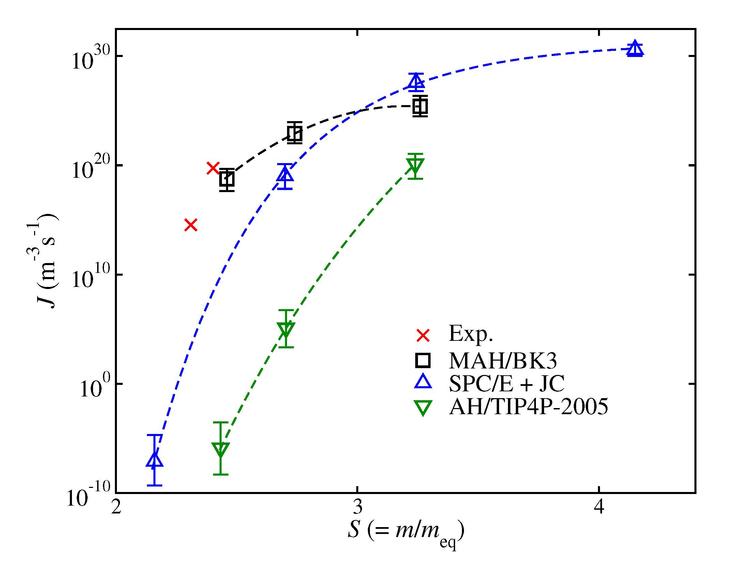
Figure from Jiang et al., J. Phys. Chem. 149: 141102 (2018), shows significant improvement in the nucleation rate prediction when polarizable models are used.

