Liquid-liquid phase separation of proteins within the intracellular environment helps organize and regulate biochemical processes necessary for biological function. We utilize grand canonical Monte Carlo simulations for a simple coarse-grained model for disordered proteins to systematically investigate how sequence distribution, hydrophobic fraction and chain length influence the phase behavior and regulate the formation of finite-size aggregates preempting macroscopic phase separation for some sequences. We show that a normalized sequence charge decoration parameter establishes a "soft" criterion for predicting the underlying phase transition of a model protein. We find that the overall phase separation propensity of sequences increases with the chain length and the hydrophobic fraction. At sufficiently long chain lengths, a vast majority of sequences are expected to phase separate. We hypothesize that this effect might contribute toward the ubiquity of phase separation and consequently, the relative rarity of aggregation behavior within cells.
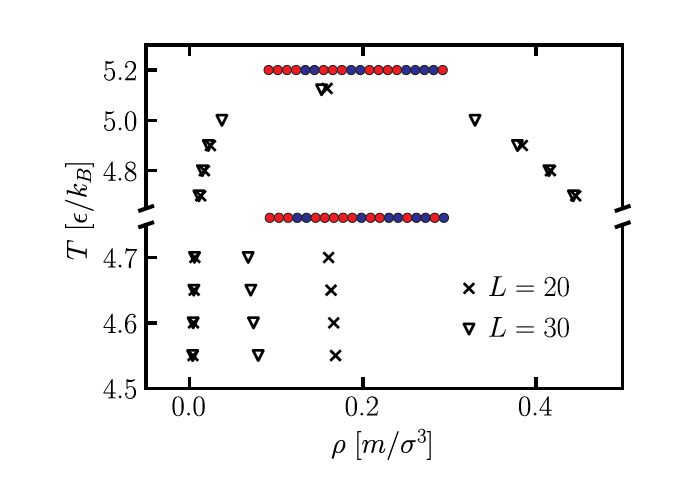
Dense and dilute phase concentrations for T4H2T3H2T4H4T (top) and T3H2T5HT2H2TH2TH (bottom) with simulations performed in systems of size L = 20 and L = 30.

